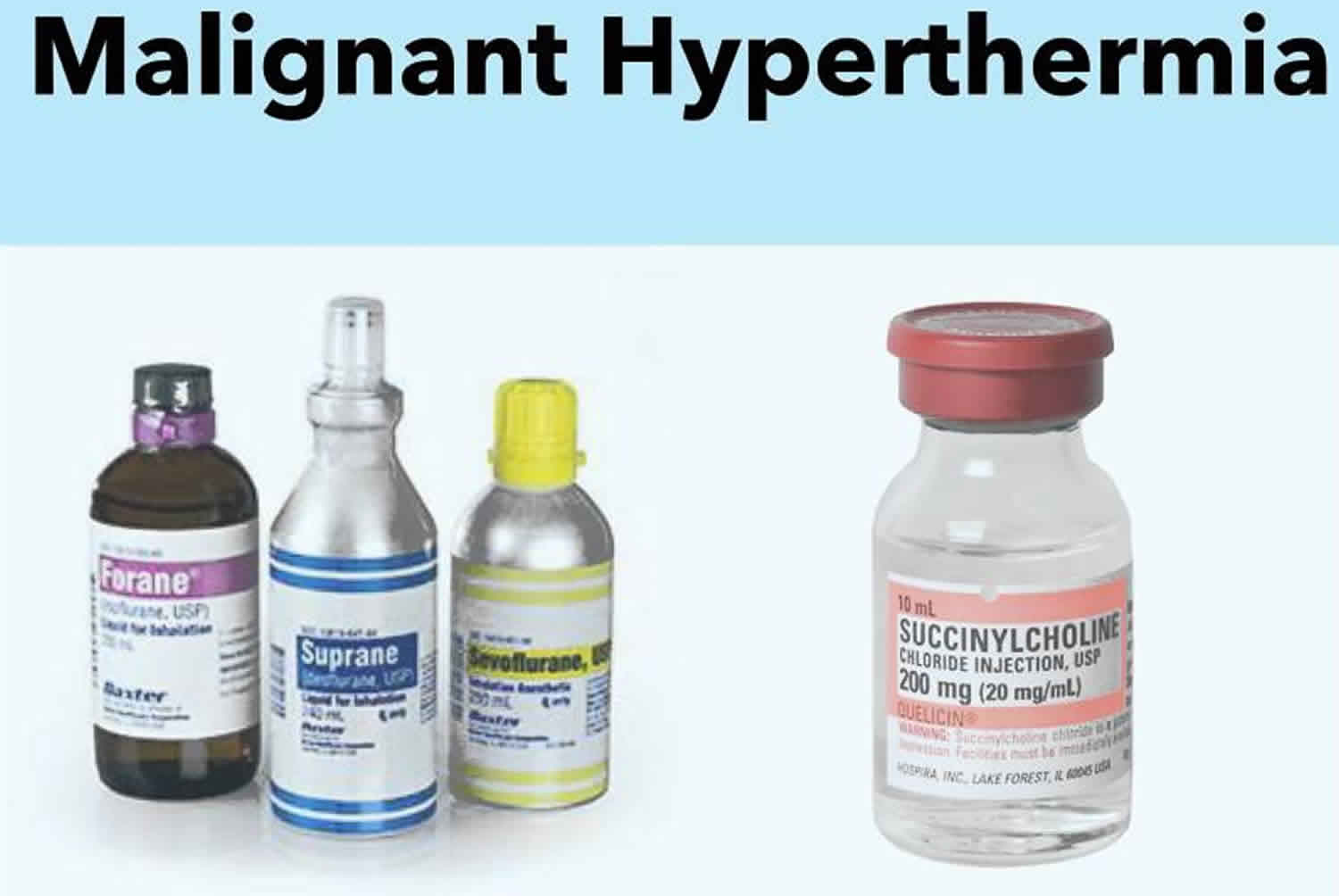
malignant hyperthermia (mh) is a pharmacogenetic disorder of skeletal muscle that presents as a hypermetabolic response to potent volatile anesthetic gases such as halothane, sevoflurane, desflurane, isoflurane and the depolarizing muscle relaxant succinylcholine, and rarely, in humans, to stressors such as vigorous exercise and heat.
If physiologic and metabolic abnormalities of mh reappear: Give an immediate intravenous (iv) bolus of dantrolene 2.5 mg/kg. Faced with a malignant hyperthermia crisis, the immediate access to sufficient dantrolene is essential to achieve the best possible outcome for the patient. The availability of dantrolene and increased intraoperative monitoring have considerably. dantrolene sodium is a postsynaptic muscle relaxant with multiple indications in the fields of anesthesiology and neurology.

malignant hyperthermia (mh) is a pharmacogenetic disorder of skeletal muscle that presents as a hypermetabolic response to potent volatile anesthetic gases such as halothane, sevoflurane, desflurane, isoflurane and the depolarizing muscle relaxant succinylcholine, and rarely, in humans, to stressors such as vigorous exercise and heat.
Whereas malignant hyperthermia association of the united states guidelines state dantrolene must be available within 10 min of the decision to treat mh wherever volatile anesthetics or succinylcholine are administered, a society for ambulatory. Treatment of malignant hyperthermia (mh): Pharmacy will have a ready available malignant hyperthermia box containing 36 dantrium (dantrolene sodium) vials. For a 70 kg adult: It is seen in response to volatile anesthetic agents and depolarizing neuromuscular blocker (nm blocker), succinylcholine. The scope of these guidelines is extended to. For paediatric dose see paediatric administration of dantrolene below. Larach mg, brandom bw, allen gc, gronert ga, lehman eb. Ryanodex® malignant hyperthermia protocol malignant hyperthermia resources malignant hyperthermia frequently asked questions. dantrolene sodium is a postsynaptic muscle relaxant with multiple indications in the fields of anesthesiology and neurology. Guidance on the clinical diagnosis of malignant hyperthermia. malignant hyperthermia (mh) is a rare, inherited skeletal muscle syndrome that presents as a. Dantrium (dantrolene sodium) will be available in the recovery room malignant hyperthermia cart, and the pharmacy department.
Larach mg, brandom bw, allen gc, gronert ga, lehman eb. However, malignant hyperthermia crises are rare, and there may be administrative pressures to limit the amount of dantrolene stocked or, in some countries, not to stock dantrolene at all. • initial bolus (2.5 mg/kg): Give dantrolene right before the procedure regardless of which agents used as anesthesia b. The dosing regimen of dantrolene should be based on actual.

It is triggered in susceptible individuals primarily by the volatile inhalational anesthetic agents and the muscle relaxant succinylcholine, though other drugs have also been implicated as potential triggers.
However, malignant hyperthermia crises are rare, and there may be administrative pressures to limit the amount of dantrolene stocked or, in some countries, not to stock dantrolene at all. For paediatric dose see paediatric administration of dantrolene below. In the treatment of malignant hyperthermia (mh), only the ryanodex® formulation of dantrolene sodium allows for rapid response with 1 vial, by 1 provider, in less than 1 minute. Of 21 patients treated with the drug, eight were judged to have unequivocal mh and were treated according to study protocol. A report from the north american malignant malignant hyperthermia (mh) is a pharmacogenetic disorder of skeletal muscle that presents as a hypermetabolic response to potent volatile anesthetic gases such as halothane, sevoflurane, desflurane, isoflurane and the depolarizing muscle relaxant succinylcholine, and rarely, in humans, to stressors such as vigorous exercise and heat. Cardiac arrests and deaths associated with malignant hyperthermia in north america from 1987 to 2006: However, malignant hyperthermia crises are rare, and there may be administrative pressures to limit the amount of dantrolene stocked or, in som … It is seen in response to volatile anesthetic agents and depolarizing neuromuscular blocker (nm blocker), succinylcholine. Repeat dosing starting with 1 mg/kg iv. 9 vials dantrolene (a total amount of 180 mg as each vial contains 20 mg and is mixed with 60 ml sterile water). For a 70 kg adult: There are no published guidelines to support.
• initial bolus (2.5 mg/kg): For a 70 kg adult: However, malignant hyperthermia crises are rare, and there may be administrative pressures to limit the amount of dantrolene stocked or, in som … malignant hyperthermia association of the united states (mhaus; Larach mg, brandom bw, allen gc, gronert ga, lehman eb.

One episode of malignant hyperthermia doesn't increase your risk for future episodes
Give dantrolene right before the procedure regardless of which agents used as anesthesia b. Sufficient dantrolene for the management of malignant hyperthermia crises. Anesthesiologists from 65 institutions participated in a multicenter study to assess the efficacy of lyophilized intravenous dantrolene sodium in treating anesthetically related malignant hyperthermia (mh). dantrolene is currently the only clinically accepted drug treatment for mh. However, malignant hyperthermia crises are rare, and there may be administrative pressures to limit the amount of dantrolene stocked or, in som … malignant hyperthermia association of the united states (mhaus; Whereas malignant hyperthermia association of the united states guidelines state dantrolene must be available within 10 min of the decision to treat mh wherever volatile anesthetics or succinylcholine are administered, a society for ambulatory. Guidance on the clinical diagnosis of malignant hyperthermia. Treatment of malignant hyperthermia (mh): It is seen in response to volatile anesthetic agents and depolarizing neuromuscular blocker (nm blocker), succinylcholine. Do not give triggering agents and monitor patient closely for signs/symptoms of malignant hyperthermia d. Cardiac arrests and deaths associated with malignant hyperthermia in north america from 1987 to 2006: Ryanodex® malignant hyperthermia protocol malignant hyperthermia resources malignant hyperthermia frequently asked questions.
38+ Dantrolene Malignant Hyperthermia Protocol Background. dantrolene sodium is a postsynaptic muscle relaxant with multiple indications in the fields of anesthesiology and neurology. malignant hyperthermia (mh) is a pharmacogenetic disorder of skeletal muscle that presents as a hypermetabolic response to potent volatile anesthetic gases such as halothane, sevoflurane, desflurane, isoflurane and the depolarizing muscle relaxant succinylcholine, and rarely, in humans, to stressors such as vigorous exercise and heat. Cardiac arrests and deaths associated with malignant hyperthermia in north america from 1987 to 2006: Guidance on the clinical diagnosis of malignant hyperthermia. Have dantrolene available and a mh crisis protocol in place.